
ELEVIDYS voices
From diagnosis to treatment to the days beyond, life with Duchenne is a journey. Here you can explore stories from others in the community about ELEVIDYS, find comfort in shared experiences, and discover different families’ perceptions in their own words.
The stories featured here include families with younger children, since treatment was initially approved for a limited age range. Now that ELEVIDYS is approved for people 4 years of age and older, we’ll be sharing more stories from the broader community. Check back often for updates!

Reach out to us if you’d like to share your story with the community.
Families have been compensated for the time required to share their story.

NARRATOR
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
Please stay tuned for additional Important Safety Information at the end of this video and full Prescribing Information at ELEVIDYS.com.
Duchenne can vary in presentation, severity, and rate of progression. This is one person’s experience and may not be representative of all individuals with Duchenne. People who receive ELEVIDYS may have different experiences and varied treatment responses. Talk to your doctor about what may be right for you or your child.
KRISTEN
Hi, my name is Kristen. This is my husband Curtis and my son Carter.
CARTER
Hello, my name is Carter.
CURTIS
Carter, he’s outgoing, very happy kid, loves to play, loves to basically mirror me in everything that I do. He likes to build things, Legos. He is a funny, funny character.
KRISTEN
Carter is full of life. He is very happy. He’s full of joy and he inspires me to live each day to the fullest and to enjoy every moment that we’re in. He’s just Mr. Happy-go-lucky and he lifts my spirits.
Carter was diagnosed with Duchenne muscular dystrophy at the age of two. When we got the genetic test back at the time, for me, it felt hopeless, and it felt very sad and heavy. And I felt crushed inside.
The first six months after diagnosis, I was in a very dark place, and I was just focused on getting through each day.
One day after work, I came home, and I was probably at my lowest low. I didn’t know who to reach out to so I went to a support group, and I typed in, “My son has Duchenne muscular dystrophy, I’m having a hard time coping.”
Within, like, a minute of me typing that post, I got an inbox on my private messenger that said, “I work with boys with DMD, and I just want to tell you that there’s a lot of hope. With this nurse that I met online, we talked the next day for about an hour. Meeting her was really important for me to really move into action.
We love our doctor but branching out and talking to another doctor was very important. So, we wanted to get a true expert’s opinion on the gene therapy drug. Once the FDA approved it, it really gave us confidence. We wanted to jump on it as fast as we could. We didn’t want to waste any time.
Once ELEVIDYS was approved, we reached out to our son's neurologist to talk about the beginning process of getting him the gene therapy. When we decided to move forward with ELEVIDYS, we got connected with SareptAssist and we had a patient coordinator that walked us through the process. It was extremely helpful to have someone facilitate what our next steps could be, what to expect from insurance, what we needed to do to prepare for the infusion.
CURTIS
We knew insurance was going to be an issue. We put in for approval for it and got denied three different times.
KRISTEN
I wasn’t going to just be okay with that. Because I’m his mother, I felt, yeah, I had to be his advocate and fight for him and get him what he needed.
CURTIS
We fought because we love our son and he means the world to us.
KRISTEN
A couple of weeks went by and one day I went to go check the mail and there was a letter in there. I opened the envelope. I saw that the decision was overturned and that was exciting.
CURTIS
Yeah, it was. Yeah, we were ecstatic to get the approval letters. It was amazing, and the next step was just, you know, getting ahold of our doctor and getting things moving.
KRISTEN
It was very important for him to get this gene therapy, and we’re going to fight for him one way or another. My advice to families, don’t take no for an answer.
CURTIS
You never give up on your kids.
KRISTEN
Since Carter has received ELEVIDYS, the impact on our family has been very positive.
CURTIS
Carter's always been a social kid, but before he was intimidated on the playground, basically just because he knew that there were things that he couldn’t do. After the infusion his walking is better, which, he's just doing awesome. I believe ELEVIDYS was the right choice for Carter. We’re grateful for it. I wish for him a joyful life.
KRISTEN
Our advice to other families is to do your research. Find a doctor that you trust, and it’s okay to see more than one doctor if you need to. If you are on the fence about getting this treatment, my advice would be to go with your gut, and to talk to a doctor, and not waste time.
NARRATOR
Please see full Prescribing Information at ELEVIDYS.com and continue watching for Important Safety Information.
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
What is the most important information to know about ELEVIDYS?
Infusion-related reactions, including hypersensitivity and serious allergic reactions (anaphylaxis), have occurred during and after ELEVIDYS infusion. Symptoms may include fast heart rate, fast breathing, swollen lips, shortness of breath, nostrils widening, hives, red and blotchy skin, itchy or inflamed lips, rash, vomiting, nausea, chills, and fever. Your doctor will monitor you during and at least 3 hours after ELEVIDYS infusion. If an infusion-related reaction occurs, your doctor may slow or stop the ELEVIDYS infusion and provide additional medical treatment as needed. Contact a healthcare provider immediately if infusion-related symptoms occur.
ELEVIDYS can increase certain liver enzyme levels and cause acute serious liver injury. Patients will receive oral corticosteroid medication before and after infusion with ELEVIDYS and will undergo weekly blood tests to monitor liver enzyme levels for 3 months after treatment. Contact a healthcare provider immediately if the patient’s skin and/or whites of the eyes appear yellowish or if the patient misses a dose of corticosteroid or vomits it up.
Administration of ELEVIDYS may be delayed in patients who have acute liver disease until the condition is resolved or under control. Patients with preexisting liver impairment, chronic liver infection, or acute liver disease may be at higher risk of acute serious liver injury.
Immune-mediated myositis (an immune response affecting muscles) was observed in patients with a deletion mutation in the DMD gene that is contraindicated.
Patients with certain mutation deletions (in exons 1 to 17 and/or exons 59 to 71) may be at risk for a severe immune-mediated myositis reaction.
Caregivers should contact a healthcare provider immediately if the patient experiences any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, breathing, or speaking, as these may be symptoms of myositis.
Myocarditis (inflammation of the heart) has been observed within days following ELEVIDYS infusion. The patient’s doctor will conduct weekly blood tests for the first month after treatment to evaluate troponin-I (a cardiac protein that can detect damage to muscle cells in the heart). Caregivers should contact a healthcare provider immediately if the patient begins to experience chest pain and/or shortness of breath. More frequent monitoring may be required if the patient has cardiac symptoms.
Patients need to have blood tests to ensure that they do not have antibodies that may prevent them from being able to receive ELEVIDYS, as introducing the gene therapy could increase the risk of a severe allergic reaction or prevent desired therapeutic levels. Treatment with ELEVIDYS is not recommended for patients who have high antibodies to the vector, the part of gene therapy used to deliver ELEVIDYS.
Due to the need to follow a corticosteroid regimen, an infection (such as cold, flu, gastroenteritis [stomach flu], otitis media [ear infection], bronchiolitis [respiratory infection], etc.) before or after ELEVIDYS infusion could lead to more serious complications. Caregivers should contact a healthcare provider immediately if they see any symptoms suggestive of infection, such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
Are there any considerations for vaccination schedules and ELEVIDYS?
Patient vaccinations should be up to date with current immunization guidelines. Vaccinations should be received at least 4 weeks prior to starting the corticosteroid regimen that is required before receiving ELEVIDYS.
Are there any precautions that need to be considered when handling a patient’s bodily waste?
Vector shedding of ELEVIDYS occurs primarily through body waste. Patients and caregivers should use proper hand hygiene, such as hand washing when coming into direct contact with patient body waste. Place potentially contaminated materials that may have the patient’s bodily fluids/waste in a sealable bag and dispose into regular trash. Precautions should be followed for 1 month after ELEVIDYS infusion.
What are the possible or likely side effects of ELEVIDYS?
The most common side effects that occurred in patients treated with ELEVIDYS were vomiting, nausea, liver injury, fever, and decreased platelet counts.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Please see full Prescribing Information at ELEVIDYS.com.
Kristen, Carter (shown here at age 4), and Curtis
"I had to become a lion for my son."
From the defeating diagnosis to the defining moment when they received the news about treatment, hear how Kristen and Curtis fought for their son at every turn.
Nathaniel | Shown here at age 6
“Having treatment with ELEVIDYS was not an easy decision — I was a nervous wreck! — but it was the right choice for Nathaniel.”
- Carolyn, Nathaniel’s mom
Alex | Shown here at age 6
“Making the decision to move forward was the hardest of my life. After educating myself and discussing it with family, I trusted my gut … every day I’m grateful I did.”
- Daylee, Alex’s mom

Charlie | Shown here at age 8

NARRATOR
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
Please stay tuned for additional Important Safety Information at the end of this video and full Prescribing Information at ELEVIDYS.com.
Duchenne can vary in presentation, severity, and rate of progression. This is one person’s experience and may not be representative of all individuals with Duchenne. People who receive ELEVIDYS may have different experiences and varied treatment responses. Talk to your doctor about what may be right for you or your child.
CHERYL
My name is Cheryl, my husband Charles and I have two boys. My son Charlie is eight years old and he is living with Duchenne muscular dystrophy. Charlie was treated as part of a clinical trial.
Charlie has a wonderful personality. He's a happy boy. He's always smiling. He's very social. He loves his big brother Noah and playing with him. He’s really smart. He loves math. He loves going to school. He loves to learn new things. He's constantly telling me things that I never knew about space or science.
CHARLES
Charlie is quite the kid. Charlie loves to explore the natural world. His mother and I are pretty dedicated into turning them into outdoor kids in a mostly indoor world.
CHERYL
Charlie was born a healthy child. As he grew, he started to miss some of his milestones. He didn't end up walking until 14 months. He fell a lot.
CHARLES
My reaction to Charlie’s diagnosis was quite simply put was disbelief. It was a very emotional period for us, for his mother and I.
Shortly after Charlie’s diagnosis, we started the work on reaching out to a variety of resources just to wrap our heads around what it meant to be diagnosed with Duchenne and what options were available for treatment.
Cheryl was really focused on connecting with families in understanding their experiences and how that might relate to us. A lot of my attention was paid to the education and the science, what the clinical trials were doing, what the data meant. You know in discussion with Charlie's neurologist, we began to understand the gene therapy candidate.
CHERYL
While we waited for Charlie to be treated with ELEVIDYS, we were really nervous. We were just anxious about it. What was going to happen that day? What if we had a reaction, just a lot of unknowns.
We had a whole big bag of activities for him to do. We had Lego sets, we had books. We had drawing pads, markers, crayons, a little screen for him to watch a movie. We had all these things ready to go for Charlie.
I was very nervous. I tried not to show it in front of Charlie. I acted really calm, but on the inside I was very nervous. When the whole thing finished, and I saw that he was okay, I could just feel the stress lift away from my body and I felt like I could breathe.
CHARLES
It was a very happy day in our household. We did have a celebration, which resulted in a very good meal that Charlie selected, if I recall. And, we just celebrated the moment. And following treatment with ELEVIDYS, we felt confident we made the right decision for him.
CHERYL
After Charlie received ELEVIDYS, he started to show some improvements at home and his teachers and aides, and even his bus driver, noticed some improvements as well.
CHARLES
My dream for Charlie is to have him accomplish everything that he wants to and be a supporter of his all along the way. No matter what the age, no matter what the circumstance, and I will do everything that I can to support him accomplishing whatever it is he seeks out to achieve.
CHERYL
Looking back, I would absolutely choose ELEVIDYS for him again.
CHARLES
We’ve been fortunate enough now, being a few years with a Duchenne diagnosis, to connect with families, some virtual, some face-to-face. And, we love telling Charlie’s story.
It’s a rare disease, you know. The community in this is small, it's concise, it's tight, but those who are involved in it are usually all in. And we're a strong part of that community.
Not all families are going to have the same experience. And, you need to expect that, but just be willing to dedicate the time and the commitment to learning everything that you can about the treatment that you’re looking at, whether it be gene therapy or otherwise.
CHERYL
Duchenne has taught our family to live life to the fullest, to get out and do the things that we want to do as a family. Make plans, have fun, just laugh and enjoy each other’s company.
My dream for Charlie is that he lives a full life and he does what he wants to do. He fulfills his dreams and makes the most of his life. I really just want Charlie to be happy and fulfilled.
NARRATOR
Please see full Prescribing Information at ELEVIDYS.com and continue watching for Important Safety Information.
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
What is the most important information to know about ELEVIDYS?
Infusion-related reactions, including hypersensitivity and serious allergic reactions (anaphylaxis), have occurred during and after ELEVIDYS infusion. Symptoms may include fast heart rate, fast breathing, swollen lips, shortness of breath, nostrils widening, hives, red and blotchy skin, itchy or inflamed lips, rash, vomiting, nausea, chills, and fever. Your doctor will monitor you during and at least 3 hours after ELEVIDYS infusion. If an infusion-related reaction occurs, your doctor may slow or stop the ELEVIDYS infusion and provide additional medical treatment as needed. Contact a healthcare provider immediately if infusion-related symptoms occur.
ELEVIDYS can increase certain liver enzyme levels and cause acute serious liver injury. Patients will receive oral corticosteroid medication before and after infusion with ELEVIDYS and will undergo weekly blood tests to monitor liver enzyme levels for 3 months after treatment. Contact a healthcare provider immediately if the patient’s skin and/or whites of the eyes appear yellowish or if the patient misses a dose of corticosteroid or vomits it up.
Administration of ELEVIDYS may be delayed in patients who have acute liver disease until the condition is resolved or under control. Patients with preexisting liver impairment, chronic liver infection, or acute liver disease may be at higher risk of acute serious liver injury.
Immune-mediated myositis (an immune response affecting muscles) was observed in patients with a deletion mutation in the DMD gene that is contraindicated.
Patients with certain mutation deletions (in exons 1 to 17 and/or exons 59 to 71) may be at risk for a severe immune-mediated myositis reaction.
Caregivers should contact a healthcare provider immediately if the patient experiences any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, breathing, or speaking, as these may be symptoms of myositis.
Myocarditis (inflammation of the heart) has been observed within days following ELEVIDYS infusion. The patient’s doctor will conduct weekly blood tests for the first month after treatment to evaluate troponin-I (a cardiac protein that can detect damage to muscle cells in the heart). Caregivers should contact a healthcare provider immediately if the patient begins to experience chest pain and/or shortness of breath. More frequent monitoring may be required if the patient has cardiac symptoms.
Patients need to have blood tests to ensure that they do not have antibodies that may prevent them from being able to receive ELEVIDYS, as introducing the gene therapy could increase the risk of a severe allergic reaction or prevent desired therapeutic levels. Treatment with ELEVIDYS is not recommended for patients who have high antibodies to the vector, the part of gene therapy used to deliver ELEVIDYS.
Due to the need to follow a corticosteroid regimen, an infection (such as cold, flu, gastroenteritis [stomach flu], otitis media [ear infection], bronchiolitis [respiratory infection], etc.) before or after ELEVIDYS infusion could lead to more serious complications. Caregivers should contact a healthcare provider immediately if they see any symptoms suggestive of infection, such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
Are there any considerations for vaccination schedules and ELEVIDYS?
Patient vaccinations should be up to date with current immunization guidelines. Vaccinations should be received at least 4 weeks prior to starting the corticosteroid regimen that is required before receiving ELEVIDYS.
Are there any precautions that need to be considered when handling a patient’s bodily waste?
Vector shedding of ELEVIDYS occurs primarily through body waste. Patients and caregivers should use proper hand hygiene, such as hand washing when coming into direct contact with patient body waste. Place potentially contaminated materials that may have the patient’s bodily fluids/waste in a sealable bag and dispose into regular trash. Precautions should be followed for 1 month after ELEVIDYS infusion.
What are the possible or likely side effects of ELEVIDYS?
The most common side effects that occurred in patients treated with ELEVIDYS were vomiting, nausea, liver injury, fever, and decreased platelet counts.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Please see full Prescribing Information at ELEVIDYS.com.
“Make a commitment to learning everything that you can about the treatment that you’re looking at, whether it be gene therapy or something else.”
- Cheryl, Charlie’s mom

“We spent a long time discussing treatment with doctors and our medical staff, which helped us feel comfortable that this would be the right choice for Max.”
Tao, dad to Max, who received treatment with ELEVIDYS
Julius | Shown here at age 6

NARRATOR
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
Please stay tuned for additional Important Safety Information at the end of this video and full Prescribing Information at ELEVIDYS.com.
Duchenne can vary in presentation, severity, and rate of progression. This is one person’s experience and may not be representative of all individuals with Duchenne. People who receive ELEVIDYS may have different experiences and varied treatment responses. Talk to your doctor about what may be right for you or your child.
SAVANNAH
My son Julius is six years old, and he lives with Duchenne muscular dystrophy.
At first it was me, my husband and Julius. For awhile there, we all got to enjoy our family of three, but it was always a goal to get my husband's four other kids. And then, during all that time, we so happened to got pregnant. Then made eight of us. Julius is very outspoken, very carefree and very funny. Naturally funny. I mean, he has me crying because he’s so funny.
MATTHEW
Julius is having the time of his life, no matter what. He’s always smiling. You know, he’s always just happy-go-lucky, funny guy. I know that my son is definitely the cornerstone of our family, a big part of our foundation, and definitely the glue that holds us together.
SAVANNAH
Muscular dystrophy is something that runs in my family. My twin brother, Derek, lived with Duchenne muscular dystrophy. I knew what the disease was like, and I wanted to take it head on as fast as I could. So, my neurologist let me know that there was a FDA-approved gene therapy that he qualified for.
My initial reaction was excitement, also a little nerve-wracking. So that kind of pushed us in weighing the pros and the cons of getting this treatment for him. My mother and sister were excited because there was opportunities available now that weren't available 20 years ago.
MATTHEW
To be honest going into ELEVIDYS, it was a little scary, but seeing that the team of doctors involved in Julius’ care, specifically his neurologist, how involved they were. How much it’s meant to them, and it wasn’t just another patient, this is somebody who was becoming a part of their family, they’re becoming a part of our family.
In the days leading up to Julius’ treatment, there was a bit of anxiety that was growing, and every day that we got closer, it was a little more anxious. You go into the hospital room or the clinic and he’s there getting his IVs and he’s doing thumbs up and I’m here, like, terrified a little bit, you know. He’s the one telling me it’s okay. He’s the one making all this possible. He’s amazing. He really is.
SAVANNAH
Today Julius is doing well. We continue to notice changes. Recently, we had a big family outing and we went to a pizza arcade. He had his brothers and sister to actually run around and play along with so that was very nice.
MATTHEW
We’re looking forward to just Julius getting to be a six-year-old boy with his brothers and his sister and play and have fun and just enjoy life.
SAVANNAH
I hope he can continue being the social butterfly that he is and continuing loving school, and loving his brothers and sisters, and just having a happy life.
MATTHEW
Our goal for Julius is for him to keep continuing to do the things he loves for as long as possible.
SAVANNAH
Since Julius was treated my feelings about Duchenne have just been more positive. We don’t know that the future holds with Duchenne, but we are happy with the path that we’re on.
MATTHEW
To think that Julius has an opportunity that his uncle didn't have is remarkable. In order to find comfort, we've come to the conclusion that people like Derek have helped lay a foundation for where we are today. Derek was a great step in allowing Julius to receive what he's received.
SAVANNAH
It’s just nice to see that my son can have the opportunities that my twin brother, unfortunately, didn’t get to have, and, you know, to see his life just be a little bit different than what my brother had to deal with.
MATTHEW
Looking back at all the circumstances that led us to ELEVIDYS and his treatment, there’s no doubt that we would decide the same decision over again.
NARRATOR
Please see full Prescribing Information at ELEVIDYS.com and continue watching for Important Safety Information.
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
What is the most important information to know about ELEVIDYS?
Infusion-related reactions, including hypersensitivity and serious allergic reactions (anaphylaxis), have occurred during and after ELEVIDYS infusion. Symptoms may include fast heart rate, fast breathing, swollen lips, shortness of breath, nostrils widening, hives, red and blotchy skin, itchy or inflamed lips, rash, vomiting, nausea, chills, and fever. Your doctor will monitor you during and at least 3 hours after ELEVIDYS infusion. If an infusion-related reaction occurs, your doctor may slow or stop the ELEVIDYS infusion and provide additional medical treatment as needed. Contact a healthcare provider immediately if infusion-related symptoms occur.
ELEVIDYS can increase certain liver enzyme levels and cause acute serious liver injury. Patients will receive oral corticosteroid medication before and after infusion with ELEVIDYS and will undergo weekly blood tests to monitor liver enzyme levels for 3 months after treatment. Contact a healthcare provider immediately if the patient’s skin and/or whites of the eyes appear yellowish or if the patient misses a dose of corticosteroid or vomits it up.
Administration of ELEVIDYS may be delayed in patients who have acute liver disease until the condition is resolved or under control. Patients with preexisting liver impairment, chronic liver infection, or acute liver disease may be at higher risk of acute serious liver injury.
Immune-mediated myositis (an immune response affecting muscles) was observed in patients with a deletion mutation in the DMD gene that is contraindicated.
Patients with certain mutation deletions (in exons 1 to 17 and/or exons 59 to 71) may be at risk for a severe immune-mediated myositis reaction.
Caregivers should contact a healthcare provider immediately if the patient experiences any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, breathing, or speaking, as these may be symptoms of myositis.
Myocarditis (inflammation of the heart) has been observed within days following ELEVIDYS infusion. The patient’s doctor will conduct weekly blood tests for the first month after treatment to evaluate troponin-I (a cardiac protein that can detect damage to muscle cells in the heart). Caregivers should contact a healthcare provider immediately if the patient begins to experience chest pain and/or shortness of breath. More frequent monitoring may be required if the patient has cardiac symptoms.
Patients need to have blood tests to ensure that they do not have antibodies that may prevent them from being able to receive ELEVIDYS, as introducing the gene therapy could increase the risk of a severe allergic reaction or prevent desired therapeutic levels. Treatment with ELEVIDYS is not recommended for patients who have high antibodies to the vector, the part of gene therapy used to deliver ELEVIDYS.
Due to the need to follow a corticosteroid regimen, an infection (such as cold, flu, gastroenteritis [stomach flu], otitis media [ear infection], bronchiolitis [respiratory infection], etc.) before or after ELEVIDYS infusion could lead to more serious complications. Caregivers should contact a healthcare provider immediately if they see any symptoms suggestive of infection, such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
Are there any considerations for vaccination schedules and ELEVIDYS?
Patient vaccinations should be up to date with current immunization guidelines. Vaccinations should be received at least 4 weeks prior to starting the corticosteroid regimen that is required before receiving ELEVIDYS.
Are there any precautions that need to be considered when handling a patient’s bodily waste?
Vector shedding of ELEVIDYS occurs primarily through body waste. Patients and caregivers should use proper hand hygiene, such as hand washing when coming into direct contact with patient body waste. Place potentially contaminated materials that may have the patient’s bodily fluids/waste in a sealable bag and dispose into regular trash. Precautions should be followed for 1 month after ELEVIDYS infusion.
What are the possible or likely side effects of ELEVIDYS?
The most common side effects that occurred in patients treated with ELEVIDYS were vomiting, nausea, liver injury, fever, and decreased platelet counts.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Please see full Prescribing Information at ELEVIDYS.com.
“Looking back at all the circumstances that led us to ELEVIDYS and his treatment, there’s no doubt that we would make the same decision over again.”
- Matthew, Julius’ dad
Jason | Shown here at age 6

NARRATOR
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
Please stay tuned for additional Important Safety Information at the end of this video and full Prescribing Information at ELEVIDYS.com.
Duchenne can vary in presentation, severity, and rate of progression. This is one person’s experience and may not be representative of all individuals with Duchenne. People who receive ELEVIDYS may have different experiences and varied treatment responses. Talk to your doctor about what may be right for you or your child.
SYLVIA
My name is Sylvia. I’m married to Robert. We have three children, so we’ve got our hands full for sure.
My son, Jason, is six and he lives with Duchenne. Jason is definitely a spitfire. He is the life of any party. And, you definitely know when he's in a room. He will make himself known for sure.
So, Jason absolutely loves dancing or singing, or even just, any type of activity that will get attention on him. I think one of the things Jason enjoys doing probably more than anything else is building Lego sets. I think that’s where he probably shines the most because, you know, he's able to be his creative self.
Once we got that Duchenne diagnosis, our family obviously had to process it. I knew immediately we needed to get the ball rolling and seeing a specialist that maybe knows a little bit more about Duchenne than possibly a doctor in our area, because we don’t have the specialty care down here.
So, we heard about ELEVIDYS through Jason's neurologist. Jason's doctor described very thoroughly what gene therapy, and specifically ELEVIDYS, would be doing. So, when they first told us about the possible side effects of ELEVIDYS, there was some nerves and some-- it wasn't doubt, but there was some moments where I was scared.
We did talk back and forth with the neurologist saying, “Hey, I have these concerns. Do you have concerns?” Once we had that lengthy conversation, we realized that this is definitely the next step for us. You know, Jason needs this gene therapy, and that’s the route we’re going to go.
Leading up to actual treatment day was kind of nerve-wracking because I was nervous. Like me, myself, I get nervous, but I would see Jason's reaction, and Jason's like, "When's my appointment? When's infusion day?" “He was excited because he goes, "I'm getting special medicine.”
He was so funny about everything and so excited about everything that it helped that process go, I think, a lot smoother then it would have been. The entire team there at the hospital were so helpful. The neurologist was so thorough in explaining the entire process of ELEVIDYS. That we were so comfortable because we knew there was going to be someone there with us every step of the way. It's all about support and we have an incredible support team when it comes to, not just his care, but anything in general.
Today, we're noticing a lot of positive changes with Jason. He's been looking forward to having his birthday party in his favorite pizza place where he can play all his arcade games with his friends and cousins.
ELEVIDYS is not a cure. I know Jason still has Duchenne. I don’t know what the future holds with Duchenne, but I’m happy with the path we’re on. Would we choose ELEVIDYS again if we were given the chance? You know, this is something that, as a family, we decided was definitely the right choice for us.
In the region that we live, some people may call it southmost Texas. You can definitely say that it’s, you know, the Latino culture, the Latino community is definitely prominent in our area. Spanish is spoken much more than English. There might be a language barrier in educating the community about Duchenne.
My advice is you need to advocate for yourself and you need to advocate for your child. If that means pushing your medical team, and asking for answers, then do that. Maybe it truly is the Latina in me where I get spicy and I just fight for what I think is right.
So, looking ahead, like looking into the future my dreams for Jason are just for him to live life to the fullest, and he honestly does that.
NARRATOR
Please see full Prescribing Information at ELEVIDYS.com and continue watching for Important Safety Information.
Important Safety Information
What is ELEVIDYS?
ELEVIDYS is a prescription gene therapy used to treat ambulatory individuals at least 4 years old with Duchenne muscular dystrophy (DMD) who have a confirmed mutation in the DMD gene.
ELEVIDYS is approved under accelerated approval for non-ambulatory patients at least 4 years old with DMD who have a confirmed mutation in the DMD gene. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. ELEVIDYS treatment increased the marker, ELEVIDYS micro-dystrophin in skeletal muscle. Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory patients with DMD.
Who should not receive ELEVIDYS?
Individuals with certain types of mutations, any deletion in exon 8 and/or exon 9 in the DMD gene, should not receive ELEVIDYS.
What is the most important information to know about ELEVIDYS?
Infusion-related reactions, including hypersensitivity and serious allergic reactions (anaphylaxis), have occurred during and after ELEVIDYS infusion. Symptoms may include fast heart rate, fast breathing, swollen lips, shortness of breath, nostrils widening, hives, red and blotchy skin, itchy or inflamed lips, rash, vomiting, nausea, chills, and fever. Your doctor will monitor you during and at least 3 hours after ELEVIDYS infusion. If an infusion-related reaction occurs, your doctor may slow or stop the ELEVIDYS infusion and provide additional medical treatment as needed. Contact a healthcare provider immediately if infusion-related symptoms occur.
ELEVIDYS can increase certain liver enzyme levels and cause acute serious liver injury. Patients will receive oral corticosteroid medication before and after infusion with ELEVIDYS and will undergo weekly blood tests to monitor liver enzyme levels for 3 months after treatment. Contact a healthcare provider immediately if the patient’s skin and/or whites of the eyes appear yellowish or if the patient misses a dose of corticosteroid or vomits it up.
Administration of ELEVIDYS may be delayed in patients who have acute liver disease until the condition is resolved or under control. Patients with preexisting liver impairment, chronic liver infection, or acute liver disease may be at higher risk of acute serious liver injury.
Immune-mediated myositis (an immune response affecting muscles) was observed in patients with a deletion mutation in the DMD gene that is contraindicated.
Patients with certain mutation deletions (in exons 1 to 17 and/or exons 59 to 71) may be at risk for a severe immune-mediated myositis reaction.
Caregivers should contact a healthcare provider immediately if the patient experiences any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, breathing, or speaking, as these may be symptoms of myositis.
Myocarditis (inflammation of the heart) has been observed within days following ELEVIDYS infusion. The patient’s doctor will conduct weekly blood tests for the first month after treatment to evaluate troponin-I (a cardiac protein that can detect damage to muscle cells in the heart). Caregivers should contact a healthcare provider immediately if the patient begins to experience chest pain and/or shortness of breath. More frequent monitoring may be required if the patient has cardiac symptoms.
Patients need to have blood tests to ensure that they do not have antibodies that may prevent them from being able to receive ELEVIDYS, as introducing the gene therapy could increase the risk of a severe allergic reaction or prevent desired therapeutic levels. Treatment with ELEVIDYS is not recommended for patients who have high antibodies to the vector, the part of gene therapy used to deliver ELEVIDYS.
Due to the need to follow a corticosteroid regimen, an infection (such as cold, flu, gastroenteritis [stomach flu], otitis media [ear infection], bronchiolitis [respiratory infection], etc.) before or after ELEVIDYS infusion could lead to more serious complications. Caregivers should contact a healthcare provider immediately if they see any symptoms suggestive of infection, such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
Are there any considerations for vaccination schedules and ELEVIDYS?
Patient vaccinations should be up to date with current immunization guidelines. Vaccinations should be received at least 4 weeks prior to starting the corticosteroid regimen that is required before receiving ELEVIDYS.
Are there any precautions that need to be considered when handling a patient’s bodily waste?
Vector shedding of ELEVIDYS occurs primarily through body waste. Patients and caregivers should use proper hand hygiene, such as hand washing when coming into direct contact with patient body waste. Place potentially contaminated materials that may have the patient’s bodily fluids/waste in a sealable bag and dispose into regular trash. Precautions should be followed for 1 month after ELEVIDYS infusion.
What are the possible or likely side effects of ELEVIDYS?
The most common side effects that occurred in patients treated with ELEVIDYS were vomiting, nausea, liver injury, fever, and decreased platelet counts.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Please see full Prescribing Information at ELEVIDYS.com.
“The entire team at the hospital was so helpful. We were comfortable because we knew there was going to be someone there with us every step of the way.”
- Sylvia, Jason’s mom
Nathaniel | Se muestra aquí a los 6 años
“Recibir tratamiento con ELEVIDYS no fue una decisión fácil. Estaba muy nerviosa, pero fue la elección correcta para Nathaniel."
- Carolyn, mamá de Nathaniel
Alex | Se muestra aquí a los 6 años
“Tomar la decisión de seguir adelante fue lo más difícil de mi vida. Después de informarme y hablarlo con mi familia, confié en mi instinto... todos los días agradezco haberlo hecho”.
- Daylee, mamá de Alex
Duchenne can vary in presentation, severity, and rate of progression. The patient stories may not be representative of all individuals with Duchenne. People who receive ELEVIDYS may have different experiences and varied treatment responses. Talk to your doctor about what might be right for you or your child.

Nathaniel, shown here at age 6
Finding light in the dark
When we heard the news of Nathaniel’s diagnosis, it was as if the world went dark. The more we learned about Duchenne, the more overwhelmed and alone we felt.
A glimmer of light appeared when our doctor shared that the FDA had approved the first gene therapy for Duchenne. There was a lot to consider as he walked us through the treatment process, but we felt so supported knowing Nathaniel would be closely monitored and our doctor would be there to answer questions. Our faith gave us additional comfort when making this difficult choice. Even our SareptAssist contact was by our side the entire time, checking in to see how we were doing and answering our many questions.
Having treatment with ELEVIDYS is not an easy decision, but it was the right choice for Nathaniel.
On treatment day, I was a nervous wreck the entire 2-hour drive to the treatment center. I knew in my heart this was the right decision, but I was scared that something would go wrong. However, when we arrived, everyone was so kind and supportive. Nathaniel was anxious about getting poked with the IV needle, but his care team gave him a teddy bear and let him put a little IV in it. Once the infusion started, the nurses brought him toys and he watched TV. We couldn’t have asked for a better care team.

Walking this path can feel daunting, but know there are others here to help you find your way. I am infinitely thankful for the many people who were with us at every step!
Carolyn, Nathaniel's mom
This story is a summary of one person’s experience and does not include all relevant information about ELEVIDYS. Please review the ELEVIDYS Treatment Guide for important information, including eligibility, safety, and monitoring.
Please see Important Safety Information and full Prescribing Information for ELEVIDYS.


Nathaniel, shown here at age 6
Finding light in the dark
When we heard the news of Nathaniel’s diagnosis, it was as if the world went dark. The more we learned about Duchenne, the more overwhelmed and alone we felt.
A glimmer of light appeared when our doctor shared that the FDA had approved the first gene therapy for Duchenne. There was a lot to consider as he walked us through the treatment process, but we felt so supported knowing Nathaniel would be closely monitored and our doctor would be there to answer questions. Our faith gave us additional comfort when making this difficult choice. Even our SareptAssist contact was by our side the entire time, checking in to see how we were doing and answering our many questions.
Having treatment with ELEVIDYS is not an easy decision, but it was the right choice for Nathaniel.
On treatment day, I was a nervous wreck the entire 2-hour drive to the treatment center. I knew in my heart this was the right decision, but I was scared that something would go wrong. However, when we arrived, everyone was so kind and supportive. Nathaniel was anxious about getting poked with the IV needle, but his care team gave him a teddy bear and let him put a little IV in it. Once the infusion started, the nurses brought him toys and he watched TV. We couldn’t have asked for a better care team.

Walking this path can feel daunting, but know there are others here to help you find your way. I am infinitely thankful for the many people who were with us at every step!
Carolyn, Nathaniel's mom
This story is a summary of one person’s experience and does not include all relevant information about ELEVIDYS. Please review the ELEVIDYS Treatment Guide for important information, including eligibility, safety, and monitoring.
Please see Important Safety Information and full Prescribing Information for ELEVIDYS.


Nathaniel, se muestra aquí a los 6 años
Encontrar luz en la oscuridad
Cuando nos enteramos del deagnóstico de Nathaniel, fue como si el mundo se oscureciera. Cuanto más conocíamos sobre la enfermedad de Duchenne, más abrumados y solos nos sentíamos.
Un rayo de luz apareció cuando nuestro médico dijo que la Administración de Alimentos y Medicamentos (Food and Drug Administration, FDA) había aprobado la primera terapia genética para Duchenne. Había mucho por considerar mientras nos explicaba el proceso de tratamiento, pero nos sentimos muy apoyados al saber que Nathaniel sería supervisado de cerca y que nuestro médico estaría allí para responder nuestras preguntas. Nuestra fe nos dio más tranquilidad a la hora de tomar esta decisión difícil. Incluso nuestro contacto de SareptAssist estuvo a nuestro lado todo el tiempo, comunicándose para ver cómo estábamos y respondiendo nuestras muchas preguntas.
Recibir tratamiento con ELEVIDYS no es una decisión fácil, pero fue la elección correcta para Nathaniel.
El día del tratamiento, estuve nerviosa durante las 2 horas que duró el trayecto hasta el centro de tratamiento. En mi corazón sabía que esta era la decisión correcta, pero tenía miedo de que algo saliera mal. Sin embargo, cuando llegamos, todos fueron muy amables y comprensivos. A Nathaniel le daba miedo que lo pincharan con la aguja intravenosa, pero su equipo de asistencia le dio un osito de peluche y dejó que le pusiera una vía intravenosa pequeña. Una vez que comenzó la infusión, el personal de enfermería le llevó juguetes y se puso a ver televisión. No pudimos haber tenido un mejor equipo de asistencia.

Recorrer este camino puede parecer abrumador, pero debe saber que aquí hay otros para ayudarlo a encontrar su camino. Estoy infinitamente agradecida por las muchas personas que nos acompañaron en cada paso.
Carolyn, mamá de Nathaniel
Esta historia es un resumen de la experiencia de una persona y no incluye toda la información relevante sobre ELEVIDYS. Revise la Guía de tratamiento de ELEVIDYS para obtener información importante, incluida la elegibilidad, la seguridad y la supervisión.
Consulte la Información importante de seguridad y la Información de prescripción completa de ELEVIDYS.


Nathaniel, se muestra aquí a los 6 años
Encontrar luz en la oscuridad
Cuando nos enteramos del deagnóstico de Nathaniel, fue como si el mundo se oscureciera. Cuanto más conocíamos sobre la enfermedad de Duchenne, más abrumados y solos nos sentíamos.
Un rayo de luz apareció cuando nuestro médico dijo que la Administración de Alimentos y Medicamentos (Food and Drug Administration, FDA) había aprobado la primera terapia genética para Duchenne. Había mucho por considerar mientras nos explicaba el proceso de tratamiento, pero nos sentimos muy apoyados al saber que Nathaniel sería supervisado de cerca y que nuestro médico estaría allí para responder nuestras preguntas. Nuestra fe nos dio más tranquilidad a la hora de tomar esta decisión difícil. Incluso nuestro contacto de SareptAssist estuvo a nuestro lado todo el tiempo, comunicándose para ver cómo estábamos y respondiendo nuestras muchas preguntas.
Recibir tratamiento con ELEVIDYS no es una decisión fácil, pero fue la elección correcta para Nathaniel.
El día del tratamiento, estuve nerviosa durante las 2 horas que duró el trayecto hasta el centro de tratamiento. En mi corazón sabía que esta era la decisión correcta, pero tenía miedo de que algo saliera mal. Sin embargo, cuando llegamos, todos fueron muy amables y comprensivos. A Nathaniel le daba miedo que lo pincharan con la aguja intravenosa, pero su equipo de asistencia le dio un osito de peluche y dejó que le pusiera una vía intravenosa pequeña. Una vez que comenzó la infusión, el personal de enfermería le llevó juguetes y se puso a ver televisión. No pudimos haber tenido un mejor equipo de asistencia.

Recorrer este camino puede parecer abrumador, pero debe saber que aquí hay otros para ayudarlo a encontrar su camino. Estoy infinitamente agradecida por las muchas personas que nos acompañaron en cada paso.
Carolyn, mamá de Nathaniel
Esta historia es un resumen de la experiencia de una persona y no incluye toda la información relevante sobre ELEVIDYS. Revise la Guía de tratamiento de ELEVIDYS para obtener información importante, incluida la elegibilidad, la seguridad y la supervisión.
Consulte la Información importante de seguridad y la Información de prescripción completa de ELEVIDYS.

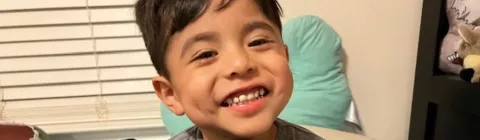
Alex, se muestra aquí a los 6 años
Una elección reflexiva
Estoy muy contenta de haber elegido ELEVIDYS para Alex; sin embargo, tomar la decisión fue lo más difícil de mi vida. Cuando su médico le recomendó ELEVIDYS por primera vez, fue difícil saber si era el camino correcto para nosotros. Alex tenía solo 5 años y me preocupaba correr un riesgo con mi dulce hijito, pero mi instinto me decía que necesitaba aprender más.

Investigué durante horas y lo hablé muchas veces con el médico de Alex. En una cita, el médico me ayudó a considerar las ventajas y desventajas, y respondió todas las preguntas a las que no tenía respuesta. La opinión de mi familia también fue importante porque siempre fueron mi mayor sistema de apoyo para Alex. Estuvieron de acuerdo con el papá de Alex, que había dicho: “Si no hacemos esto, siempre nos preguntaremos lo que podría haber sido”. Aunque estaba preocupada, como madre, tuve que seguir mi instinto y seguir adelante con el tratamiento.
Después de informarme y hablarlo con mi familia, confié en mi instinto y elegí ELEVIDYS.
Cuando llegó el día del tratamiento, estaba muy nerviosa. Respiré hondo muchas veces, abracé mucho a Alex y confié en mí misma.

Hoy, 9 meses después del tratamiento, estoy muy contenta con el camino que elegí y agradecida con las personas que me animaron a pensar en todas las posibilidades. Elegir ELEVIDYS no fue fácil, pero todos los días estoy agradecida de haberlo hecho.
Esta historia es un resumen de la experiencia de una persona y no incluye toda la información relevante sobre ELEVIDYS. Revise la Guía de tratamiento de ELEVIDYS para obtener información importante, incluida la elegibilidad, la seguridad y la supervisión.
Consulte la Información importante de seguridad y la Información de prescripción completa de ELEVIDYS.


Alex, se muestra aquí a los 6 años
Una elección reflexiva
Estoy muy contenta de haber elegido ELEVIDYS para Alex; sin embargo, tomar la decisión fue lo más difícil de mi vida. Cuando su médico le recomendó ELEVIDYS por primera vez, fue difícil saber si era el camino correcto para nosotros. Alex tenía solo 5 años y me preocupaba correr un riesgo con mi dulce hijito, pero mi instinto me decía que necesitaba aprender más.

Investigué durante horas y lo hablé muchas veces con el médico de Alex. En una cita, el médico me ayudó a considerar las ventajas y desventajas, y respondió todas las preguntas a las que no tenía respuesta. La opinión de mi familia también fue importante porque siempre fueron mi mayor sistema de apoyo para Alex. Estuvieron de acuerdo con el papá de Alex, que había dicho: “Si no hacemos esto, siempre nos preguntaremos lo que podría haber sido”. Aunque estaba preocupada, como madre, tuve que seguir mi instinto y seguir adelante con el tratamiento.
Después de informarme y hablarlo con mi familia, confié en mi instinto y elegí ELEVIDYS.
Cuando llegó el día del tratamiento, estaba muy nerviosa. Respiré hondo muchas veces, abracé mucho a Alex y confié en mí misma.

Hoy, 9 meses después del tratamiento, estoy muy contenta con el camino que elegí y agradecida con las personas que me animaron a pensar en todas las posibilidades. Elegir ELEVIDYS no fue fácil, pero todos los días estoy agradecida de haberlo hecho.
Esta historia es un resumen de la experiencia de una persona y no incluye toda la información relevante sobre ELEVIDYS. Revise la Guía de tratamiento de ELEVIDYS para obtener información importante, incluida la elegibilidad, la seguridad y la supervisión.
Consulte la Información importante de seguridad y la Información de prescripción completa de ELEVIDYS.

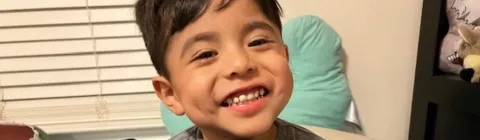
Alex, shown here at age 6
A thoughtful choice
I’m so happy I chose ELEVIDYS for Alex; however, making the decision was the hardest of my life. When his doctor first recommended ELEVIDYS, it was difficult to know if it was the right path for us. Alex was only 5 years old, and I was worried I’d be taking a risk with my sweet little boy, but my gut was telling me I needed to learn more.

I did hours of research and talked it through with Alex’s doctor many times. At one appointment, she helped me weigh the pros and cons and answered all my questions I did not have answers to. My family’s opinion was also important because they have always been my biggest support system for Alex. They agreed with Alex’s dad, who had said, “If we don’t do this, we will always wonder what could have been.” Even though I was worried, as a mom, I had to follow my gut and move forward with treatment.
After educating myself and discussing it with family, I trusted my gut and chose ELEVIDYS.
When treatment day came, I was so nervous. I took many deep breaths, gave Alex lots of hugs, and trusted myself.

Today, 9 months after treatment, I am so happy with the path I chose, and grateful to the people who encouraged me to think through all the possibilities. Choosing ELEVIDYS was not easy, but every day I am grateful I did.
Daylee, Alex’s mom
This story is a summary of one person’s experience and does not include all relevant information about ELEVIDYS. Please review the ELEVIDYS Treatment Guide for important information, including eligibility, safety, and monitoring.
Please see Important Safety Information and full Prescribing Information for ELEVIDYS.


Alex, shown here at age 6
A thoughtful choice
I’m so happy I chose ELEVIDYS for Alex; however, making the decision was the hardest of my life. When his doctor first recommended ELEVIDYS, it was difficult to know if it was the right path for us. Alex was only 5 years old, and I was worried I’d be taking a risk with my sweet little boy, but my gut was telling me I needed to learn more.

I did hours of research and talked it through with Alex’s doctor many times. At one appointment, she helped me weigh the pros and cons and answered all my questions I did not have answers to. My family’s opinion was also important because they have always been my biggest support system for Alex. They agreed with Alex’s dad, who had said, “If we don’t do this, we will always wonder what could have been.” Even though I was worried, as a mom, I had to follow my gut and move forward with treatment.
After educating myself and discussing it with family, I trusted my gut and chose ELEVIDYS.
When treatment day came, I was so nervous. I took many deep breaths, gave Alex lots of hugs, and trusted myself.

Today, 9 months after treatment, I am so happy with the path I chose, and grateful to the people who encouraged me to think through all the possibilities. Choosing ELEVIDYS was not easy, but every day I am grateful I did.
Daylee, Alex’s mom
This story is a summary of one person’s experience and does not include all relevant information about ELEVIDYS. Please review the ELEVIDYS Treatment Guide for important information, including eligibility, safety, and monitoring.
Please see Important Safety Information and full Prescribing Information for ELEVIDYS.




